| Issue |
J. Phys. I France
Volume 6, Number 12, December 1996
|
|
|---|---|---|
| Page(s) | 1555 - 1565 | |
| DOI | https://doi.org/10.1051/jp1:1996173 | |
J. Phys. I France 6 (1996) 1555-1565
Stable Molecular Metal [ Pd(dddt)
 ] Ag
] Ag
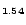 Br
Br
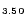 : Synthesis, Crystal Structure, Transport Properties and Electronic Band Structure
: Synthesis, Crystal Structure, Transport Properties and Electronic Band Structure
L.A. Kushch1, S.V. Konovalikhin1, L.I. Buravov1, A.G. Khomenko1, G.V. Shilov1, K. Van2, O.A. Dyachenko1, E. B. Yagubskii1, C. Rovira3 and E. Canadell4, 5
1 Institute of Chemical Physics in Chernogolovka, Russian Academy of Sciences, Chernogolovka MD, 142432 Russia
2 Institute of Experimental Mineralogy, Russian Academy of Sciences, Chernogolovka MD, 142432 Russia
3 Deparament de Quìmica Física, Facultat de Quìmica, Universitat de Barcelona, 08028 Barcelona, Spain
4 Institut de Ciencia de Materials de Barcelona (CSIC), Campus de la UAB, 08193 Bellaterra, Spain
5 Laboratoire de Structure et Dynamique des Systèmes Moléculaires et Solides, Université de Montpellier II, 34095 Montpellier Cedex, France
(Received 5 March 1996, accepted 11 June 1996)
Abstract
The synthesis, crystal and electronic band structures as well as conducting properties of the new stable molecular metal [
Pd(dddt)
2] Ag
1.54Br
3.50 (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolato) are reported. the crystal structure contains layers of donor cations alternating
with layers of silver bromide complex anions along the
a axis of the unit cell. The Ag and Br atoms are disordered in the anion layer. The conducting layers contain uniform stacks
of the translationally equivalent Pd(dddt)
2 cations along the
c-axis with a Pd
...Pd distance of 4.157(2) Å. Within the cation layers there are shortened interstack S
...S contacts (3.49(3) and 3.56(3) Å). The temperature dependence of the resistivity exhibits metallic behaviour down to 1.3 K.
The resistivity anisotropy (
![]() ) at room temperature is about 600 and does not change considerably when decreasing the temperature down to 4.2 K. The origin
of the metallic conductivity of [ Pd(dddt)
2] Ag
1.54Br
3.50 as well as the stability of this salt with respect to metal-to-insulator transitions is explained on the basis of tight binding
band structure calculations.
) at room temperature is about 600 and does not change considerably when decreasing the temperature down to 4.2 K. The origin
of the metallic conductivity of [ Pd(dddt)
2] Ag
1.54Br
3.50 as well as the stability of this salt with respect to metal-to-insulator transitions is explained on the basis of tight binding
band structure calculations.
© Les Editions de Physique 1996



